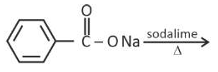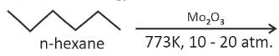Your Performance Summary!
Questions Available: 9
Questions Attempted: 0
Number of Attempts: 0
Correct Attempts: 0
Total Time Spent: 00:00
Avg Time Per Question: 00:00
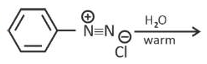
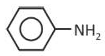
Solution
Year: 2024
Topic: Hydrocarbons
2. Given below are two statements:
Statement I: The boiling point of three isomeric pentanes follows the order n-pentane > isopentane > neopentane
Statement II: When branching increases, the molecule attains a shape of sphere. This results in smaller surface area for contact, due to which the intermolecular forces between the spherical molecules are weak thereby lowering the boiling point. In the light of the above statements, Choose the most appropriate answer form the options given below
Statement I: The boiling point of three isomeric pentanes follows the order n-pentane > isopentane > neopentane
Statement II: When branching increases, the molecule attains a shape of sphere. This results in smaller surface area for contact, due to which the intermolecular forces between the spherical molecules are weak thereby lowering the boiling point. In the light of the above statements, Choose the most appropriate answer form the options given below
(1).Statement I is correct but Statement II is incorrect
(2). Statement I is incorrect but Statement II is correct
(3). Both Statement I and Statement II are correct
(4). Both Statement I and Statement II are incorrect

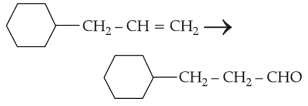



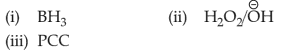
 Choose the correct answer from the options given below :
Choose the correct answer from the options given below :