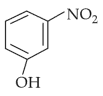
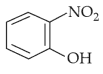
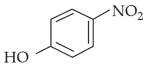
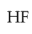Your Performance Summary!
Questions Available: 8
Questions Attempted: 0
Number of Attempts: 0
Correct Attempts: 0
Total Time Spent: 00:00
Avg Time Per Question: 00:00
Solution
Solution
Year: 2024
Topic: Structure of Atom
3. Given below are two statements:
Statement I: The boiling point of hygrates of Group 16 elements follow the order
\(\text{H}_2\text{O}\,>\,\text{H}_2\text{Te}\,>\,\text{H}_2\text{Se}\,>\,\text{H}_2\text{S}\)
Statement II: On the basis of molecular mass, \(\text{H}_2\text{O}\) is expected to have lower boiling point than the other members of the group but due to the presence of extensive H-bonding in \(\text{H}_2\text{O}\), it has higher boiling point.
Statement I: The boiling point of hygrates of Group 16 elements follow the order
\(\text{H}_2\text{O}\,>\,\text{H}_2\text{Te}\,>\,\text{H}_2\text{Se}\,>\,\text{H}_2\text{S}\)
Statement II: On the basis of molecular mass, \(\text{H}_2\text{O}\) is expected to have lower boiling point than the other members of the group but due to the presence of extensive H-bonding in \(\text{H}_2\text{O}\), it has higher boiling point.
(1).Statement I is true, but Statement II is false.
(2). Statement I is false, but Statement II is true..
(3). Both Statement I and Statement II are true.
(4). Both Statement I and Statement II are false.
Solution
Year: 2024
Topic: Structure of Atom
4. The energy of an electron in the ground state (n = 1) for \(\text{He}^+\) ion is \(-x\text{J}\), then that for an electron in n = 2 state for \(\text{Be}^{3+}\) ion in J is:
(1).\(\displaystyle-\,4x\)
(2). \(\displaystyle-\,\frac{4}{9}x\)
(3). \(\displaystyle-\,x\)
(4). \(\displaystyle-\,\frac{x}{9}\)
Solution
Solution
Year: 2025
Topic: Structure of Atom
6. The ratio of the wavelengths of the light absorbed by a Hydrogen atom when it undergoes n = 2 → n = 3 and n = 4 → n = 6 transitions, respectively, is
(1).\(\displaystyle \frac{1}{4}\)
(2). \(\displaystyle \frac{1}{36}\)
(3). \(\displaystyle \frac{1}{16}\)
(4). \(\displaystyle \frac{1}{9}\)
Solution
Solution
Year: 2025
Topic: Structure of Atom
8. Energy and radius of first Bohr orbit of He+ and Li2+ are
[Given RH = 2.18 × 10-18 J, ao = 52.9 pm]
[Given RH = 2.18 × 10-18 J, ao = 52.9 pm]
(1).\(E_n\left(Li^{2+}\right)\, =\, -8.72 \times 10^{-16}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 17.6\, pm\)
\(E_n\left(He^{+}\right)\, =\, -19.62 \times 10^{-16}\, J\);
\(r_n\left(He^{+}\right)\, =\, 17.6\, pm\)
(2). \(E_n\left(Li^{2+}\right)\, =\, -19.62 \times 10^{-18}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 17.6\, pm\)
\(E_n\left(He^{+}\right)\, =\, -8.72 \times 10^{-18}\, J\);
\(r_n\left(He^{+}\right)\, =\, 26.4\, pm\)
(3). \(E_n\left(Li^{2+}\right)\, =\, -8.72 \times 10^{-18}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 26.4\, pm\)
\(E_n\left(He^{+}\right)\, =\, -19.62 \times 10^{-18}\, J\);
\(r_n\left(He^{+}\right)\, =\, 17.6\, pm\)
(4). \(E_n\left(Li^{2+}\right)\, =\, -19.62 \times 10^{-16}\, J\);
\(r_n\left(Li^{2+}\right)\, =\, 17.6\, pm\)
\(E_n\left(He^{+}\right)\, =\, -8.72 \times 10^{-16}\, J\);
\(r_n\left(He^{+}\right)\, =\, 26.4\, pm\)