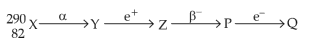Your Performance Summary!
Questions Available: 19
Questions Attempted: 0
Number of Attempts: 0
Correct Attempts: 0
Total Time Spent: 00:00
Avg Time Per Question: 00:00
Year: 2021
Topic: Nuclei
1. A radioactive nucleus \({}^{A}_{z}X\) undergoes spontaneous decay inthe sequence
\({}^{A}_{z}X \, \rightarrow \, {}_{z-1}B \, \rightarrow \, {}_{z-3}C \, \rightarrow \, {}_{z-2}D\) where Z is the atomic number of element X. The possible decay particles in the sequence are
\({}^{A}_{z}X \, \rightarrow \, {}_{z-1}B \, \rightarrow \, {}_{z-3}C \, \rightarrow \, {}_{z-2}D\) where Z is the atomic number of element X. The possible decay particles in the sequence are
(1).\(\alpha,\, \beta^-,\, \beta^+\)
(2). \(\alpha,\, \beta^+,\, \beta^-\)
(3). \(\beta^+,\, \alpha,\, \beta^-\)
(4). \(\beta^-,\, \alpha,\, \beta^+\)
Solution
Year: 2021
Topic: Nuclei
2. The half-life of a radioactive nuclide is 100 h. The fraction of original activity that will remainafter 150 h would be
(1).\(\displaystyle \frac{1}{2}\)
(2). \(\displaystyle \frac{1}{2 \sqrt{2}}\)
(3). \(\displaystyle \frac{2}{3}\)
(4). \(\displaystyle \frac{2}{3 \sqrt{2}}\)
Solution
Year: 2020 October
Topic: Nuclei
3. What happens to themass number and atomic number of an element when it emits \(\gamma\) -radiation?
(1).Mass number decreases by four andatomic number decreases by two.
(2). Mass number and atomic numberremain unchanged.
(3). Mass number remains unchanged,while atomic number decreases byone.
(4). Mass number increases by four andatomic number increases by two.
Solution
Solution
Solution
Solution
Solution
Year: 2017
Topic: Nuclei
8. Radioactive material A has decay constant \(8 \lambda\) and material B has decay constant \(\lambda\). Initially, they have same number of nuclei. After what time, the ratio of number ofnuclei of material B to that A will be 1\e ?
(1).\(\displaystyle \frac{1}{\lambda}\)
(2). \(\displaystyle \frac{1}{7 \lambda}\)
(3). \(\displaystyle \frac{1}{8\lambda}\)
(4). \(\displaystyle \frac{1}{9\lambda}\)
Solution
Solution
Year: 2021
Topic: Nuclei
10. A nucleus with mass number 240 breaks into two fragments each of mass number 120, the binding energy per nucleon of unfragmented nuclei is 7.6 MeV while that of fragments is 8.5 MeV. The total gain in the binding energyin the process is
(1).0.9 MeV
(2). 9.4 MeV
(3). 804 MeV
(4). 216 MeV
Solution
Solution
Year: 2020 September
Topic: Nuclei
12. When a uraniumisotope \(\displaystyle {}^{235}_92U\) is bombarded with a neutron, itgenerates \(\displaystyle {}^{89}_{36}Kr\), three neutrons and
(1).\(\displaystyle {}^{91}_{40}Zr\)
(2). \(\displaystyle {}^{101}_{36}Kr\)
(3). \(\displaystyle {}^{103}_{36}Kr\)
(4). \(\displaystyle {}^{144}_{56}Ba\)
Solution
Year: 2014
Topic: Nuclei
13. The binding energy per nucleon of \(_3Li^7\) and \(_2He^4\) nuclei are 5.60 MeV and 7.06 MeV, respectively. In the nuclear reaction \(_3Li^7 + _1H^1 \rightarrow _2He^2 + _2He^4 + Q\), the value of energy Q released is
(1).19.6MeV
(2). -2.4 MeV
(3). 8.4 MeV
(4). 17.3 MeV
Solution
Year: 2014
Topic: Nuclei
14. A radio isotope X with a half life 1.4 x 10sup9 yr decays of Y which is stable. A sample of the rock from a cave was found to contain X and Y in teh ratio 1:7. The age of the rock is
(1).1.96 x 109 yr
(2). 3.92 x 109 yr
(3). 4.20 x 109 yr
(4). 8.40 x 109 yr
Solution
Solution
Solution
Year: 2022
Topic: Nuclei
17. In the given nuclear reaction, the element X is :
\(\displaystyle ^{23} _{11} \,Na \rightarrow\,X+e^+ + v\)
\(\displaystyle ^{23} _{11} \,Na \rightarrow\,X+e^+ + v\)
(1).\(\displaystyle ^{23} _{11} \,Na \)
(2). \(\displaystyle ^{23} _{10} \,Ne \)
(3). \(\displaystyle ^{22} _{10} \,Ne \)
(4). \(\displaystyle ^{22} _{12} \,Mg \)
Solution
Solution
Year: 2015
Topic: Nuclei
19. If radius of the \(_{13}\text{Al}^{27}\) nucleus is taken to be \(R_{\text{Al}}\), then the radius of \(_{53}\text{Te}^{125}\) nucleus is nearly
(1).\(\displaystyle \left(\frac{53}{13} \right)^{\frac{1}{3}} R_{Al}\)
(2). \(\displaystyle \frac{5}{3}R_{\text{Al}}\)
(3). \(\displaystyle \frac{3}{5}R_{\text{Al}}\)
(4). \(\displaystyle \frac{13}{53}R_{\text{Al}}\)