Your Performance Summary!
Questions Available: 16
Questions Attempted: 0
Number of Attempts: 0
Correct Attempts: 0
Total Time Spent: 00:00
Avg Time Per Question: 00:00
Solution
Year: 2020 October
Topic: Kinetic Theory
2. An ideal gas equation can bewritten as \( p = \frac{ \rho RT}{M_0}\)where, \(\rho\) and \(M_0\) are respectively,
(1).mass density, mass of the gas
(2). number density, molar mass
(3). mass density, molar mass
(4). number density, mass of the gas
Solution
Year: 2020 September
Topic: Kinetic Theory
3. The mean free path l for a gas,with molecular diameter d and number density n can be expressed as
(1).\(\frac{1}{\sqrt{2}n\pi d^2}\)
(2). \(\frac{1}{\sqrt{2}n^2\pi d^2}\)
(3). \(\frac{1}{\sqrt{2}n^2\pi^2 d^2}\)
(4). \(\frac{1}{\sqrt{2}n\pi d}\)
Solution
Solution
Solution
Year: 2018
Topic: Kinetic Theory
6. At what temperature will the rms speed of oxygen molecules become just sufficient for escaping from the Earth’s atmosphere? (Given: mass of oxygen molecule,\(m = 2.76 × 10^−{26}\, kg\) ,Boltzmann’s constant\(k_B = 1.38 10^{-23} J K^{ −1}\))
(1).\(5.016 × 10^4\, K\)
(2). \(8.326 × 10^4\, K\)
(3). \(2.508 × 10^4\, K\)
(4). \(1.254 × 10^4\, K\)
Solution
Year: 2016
Topic: Kinetic Theory
7. The molecules of a given mass of a gas have r.m.s. velocity of \(200ms^{−1}\) at \(27^\circ C\) and \(1.0 × 10^5 Nm^{ −2}\) pressure. When the temperature and pressure of the gas are respectively, \(127^ \circ C\) and 0\(.05 × 10^5 Nm^{ −2}\) , the rms velocity of itsmolecules in \(ms^{ −1}\) is
(1).\(\frac{400}{\sqrt{3}}\)
(2). \(\frac{100\sqrt{2}}{3}\)
(3). \(\frac{100}{3}\)
(4). \(100\sqrt{2}\)
Solution
Solution
Solution
Solution
Year: 2019 Odisha
Topic: Kinetic Theory
11. The value of \(\gamma =\left(\frac{C_p}{C_v}\right)\), for hydrogen, helium and another ideal diatomic gas X (whose molecules are not rigid but have an additional vibrational mode), are respectively equal to
(1).7/5, 5/3, 9/7
(2). 5/3, 7/5, 9/7
(3). 5/3, 7/5, 7/5
(4). 7/5, 5/3, 7/5
Solution
Solution
Solution
Solution
Year: 2024
Topic: Kinetic Theory
15. The following graph represents the T - V curves of an ideal gas ( where T is the temperature and V the volume ) at three pressures P1 , P2 and P3 compared with those of Charles's law represented as dotted lines .
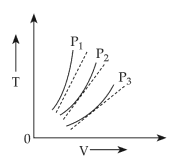

(1).P2 > P1 > P3
(2). P1 > P2 > P3
(3). P3 > P2 > P1
(4). P1 > P3 > P2
Solution
Year: 2015
Topic: Kinetic Theory
16. The ratio of specific heats \(\displaystyle \frac{C_p}{C_v}\,=\, \gamma\) in terms of degrees of freedom (n) is given by:
(1).\(\displaystyle \left(1\,+\,\frac{1}{n} \right)\)
(2). \(\displaystyle \left(1\,+\,\frac{n}{3} \right)\)
(3). \(\displaystyle \left(1\,+\,\frac{2}{n} \right)\)
(4). \(\displaystyle \left(1\,+\,\frac{n}{2} \right)\)